Analytical Methods for Amino Acids in Ginseng
Analytical Methods for Amino Acids in Ginseng
Ginseng is rich in various amino acids, most of which are essential for the human body. The amino acid content has been established as one of the key indicators for evaluating the quality of ginseng products. Current methods for determining amino acids in ginseng primarily include amino acid auto-analyzers and derivatization reversed-phase high-performance liquid chromatography (HPLC). The former suffers from drawbacks such as complex instrumentation, large size, and high costs, while the latter requires extensive optimization of derivatization reagents, reaction conditions, and product stability, making it unsuitable for direct and rapid amino acid determination. This study introduces, for the first time, a method utilizing high-performance liquid chromatography coupled with evaporative light scattering detection (HPLC-ELSD) for the direct determination of amino acids in ginseng.

Materials and Instruments
Ginseng Samples
Fresh ginseng was collected from major production regions in Jilin Province and Heilongjiang Province (China), as well as from North Korea and South Korea. Detailed information is provided in the Appendix.
Instruments
- Shimadzu LC-10AT liquid chromatograph (Shimadzu Corporation, Japan)
- Shimadzu CBM-102 chromatography workstation (Shimadzu Corporation, Japan)
- SEDERE SEDEX 75 evaporative light scattering detector (SEDERE, France)
- AT-330 column thermostat (Tianjin Aotesaisi Instrument Co., Ltd., China)
- R201D constant-temperature water bath (Shanghai Yukang Science and Education Instrument Equipment Co., Ltd., China)
- FA1104N electronic analytical balance (Shangqinghua Technology Instrument Co., Ltd., China)
- FW177 high-speed universal grinder (Beijing Yongguangming Medical Instrument Co., Ltd., China)
- RCT-3200 ultrapure water system (Changchun Labopat Technology Development Co., Ltd., China)
- GZX-9076MBE digital blast drying oven (Shanghai Boxun Industrial Co., Ltd., China)
- High-purity nitrogen gas
Reagents
- Acetonitrile (chromatographic grade, Fisher Scientific, USA)
- Methanol (chromatographic grade, Fisher Scientific, USA)
- Ultrapure water
- Trifluoroacetic acid (TFA, purity >99.5%, Xiya Reagent Co., China)
- Heptafluorobutyric acid (purity >98%, Aladdin Reagent, analytical grade)
- 24 amino acid reference standards (purity >99.0%, BDH Chemicals, UK):
- Alanine (Ala)
- 2-Aminobutyric acid
- Arginine (Arg)
- Phenylalanine (Phe)
- Aspartic acid (Asp)
- Cysteine (Cys)
- Cystine (Cys-Cys)
- DL-3-(3,4-Dihydroxyphenyl)alanine (DL-3,4-DOPA)
- Glutamic acid (Glu)
- Glycine (Gly)
- Histidine (His)
- L-Hydroxyproline
- Leucine (Leu)
- Isoleucine (Ile)
- Norleucine (Nle)
- Lysine (Lys)
- Methionine (Met)
- Tryptophan (Trp)
- Ornithine (Orn)
- Proline (Pro)
- Serine (Ser)
- Threonine (Thr)
- Tyrosine (Tyr)
- Valine (Val)
Experimental Methods
Preparation of Reference Mixed Solutions and Test Sample Solutions
Preparation of Reference Mixed Solutions**
Accurately weigh appropriate amounts of 23 amino acid reference standards (excluding cystine), including histidine, and dissolve them in 0.01 mol/L hydrochloric acid in a single volumetric flask to prepare Reference Mixed Solution I with each amino acid concentration approximately 1.0 g/L. Separately, accurately weigh an appropriate amount of cystine reference standard into another volumetric flask, dissolve it in 0.1 mol/L hydrochloric acid, and dilute to volume with ultrapure water to prepare Reference Solution II with cystine concentration approximately 1.0 g/L. Mix equal volumes of these two solutions before use to prepare Mixed Reference Solution A (24 amino acids, 0.5 g/L). Reserve for later use.
Accurately weigh appropriate amounts of aspartic acid, glycine, serine, hydroxyproline, alanine, glutamic acid, ornithine, and cysteine in a single volumetric flask, dissolve them in 0.01 mol/L hydrochloric acid to prepare Reference Mixed Solution III with each amino acid concentration approximately 1.0 g/L. Mix equal volumes of Solutions II and III to prepare Mixed Reference Solution B (0.5 g/L). Reserve for later use.
Preparation of Test Sample Solutions
Weigh 0.1 g of ginseng sample powder (passed through a 100-mesh sieve), accurately measured, into a stoppered conical flask. Add 20 mL of 6 mol/L hydrochloric acid, purge with nitrogen gas, seal, and hydrolyze at 110°C for 24 hours. Cool to room temperature, filter, evaporate the filtrate to dryness, transfer and dilute to volume with ultrapure water in a 5 mL volumetric flask, mix thoroughly, and filter through a 0.45 μm microporous membrane. Collect the filtrate and reserve for analysis.
Chromatographic Conditions
Column: Spursi i Tc MCB chromatographic column (250 mm × 4.6 mm, 5 μm).
For Mixed Reference Solution A:
- Mobile Phase A: Acetonitrile : Methanol = 1 : 1
- Mobile Phase B: 0.03% trifluoroacetic acid (TFA) solution (containing 5 mmol/L heptafluorobutyric acid).
- Gradient elution program:
0–15 min: 0% Mobile Phase A (hold until 65 min);
30 min: increase to 15% Mobile Phase A (hold at 15% until 50 min);
66 min: return to 0% Mobile Phase A (hold until 76 min). - Column temperature: 25°C; Drift tube temperature: 40°C; Nitrogen flow rate: 2.9 L/min; Injection volume: 20 μL.
For Mixed Reference Solution B:
- Mobile Phase: 0.5% TFA solution (containing 5 mmol/L heptafluorobutyric acid).
- Flow rate: 0.5 mL/min; Column temperature: 25°C; Drift tube temperature: 40°C; Nitrogen flow rate: 2.9 L/min; Injection volume: 15 μL.
Total Amino Acid Content Determination Method
Construction of Standard Curves
Accurately inject 4 μL, 6 μL, 8 μL, 10 μL, 12 μL, 14 μL, and 16 μL of Mixed Reference Solution A into the HPLC-ELSD system under the chromatographic conditions described above. Plot the natural logarithm of the injected mass (μg) of each amino acid against the corresponding peak area for linear regression analysis.
Instruments
- Shimadzu LC-10AT liquid chromatograph (Shimadzu Corporation, Japan)
- Shimadzu CBM-102 chromatography workstation (Shimadzu Corporation, Japan)
- SEDERE SEDEX 75 evaporative light scattering detector (SEDERE, France)
- AT-330 column thermostat (Tianjin Aotesaisi Instrument Co., Ltd., China)
- R201D constant-temperature water bath (Shanghai Yukang Science and Education Instrument Equipment Co., Ltd., China)
- FA1104N electronic analytical balance (Shangqinghua Technology Instrument Co., Ltd., China)
- FW177 high-speed universal grinder (Beijing Yongguangming Medical Instrument Co., Ltd., China)
- RCT-3200 ultrapure water system (Changchun Labopat Technology Development Co., Ltd., China)
- GZX-9076MBE digital blast drying oven (Shanghai Boxun Industrial Co., Ltd., China)
- High-purity nitrogen gas
Tab 1.1 Regression equations with correlation coefficients (R) of twenty-four amino acids
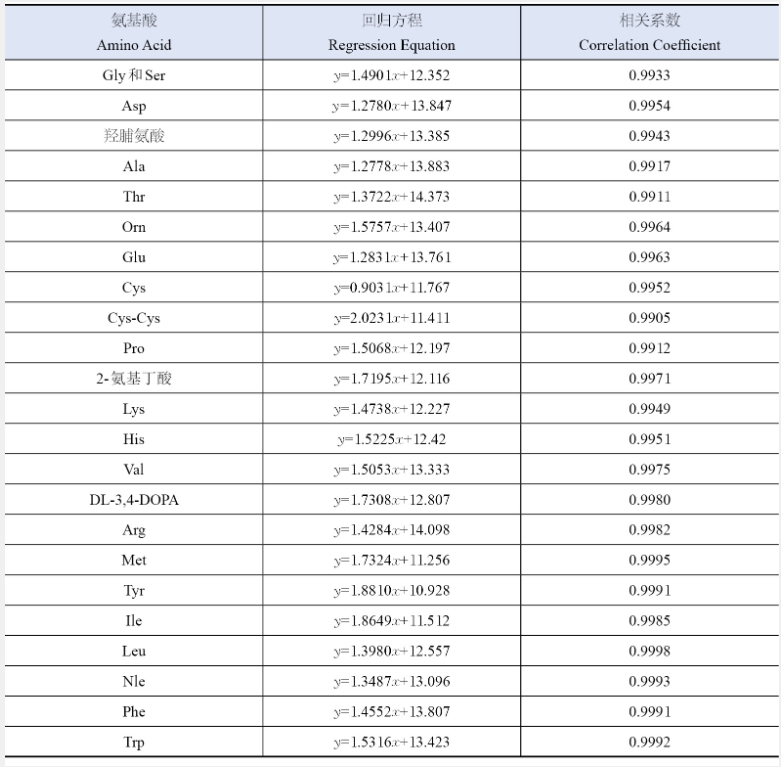
ynatural logarithm of peak area, xnatural logarithm of mass (ug)
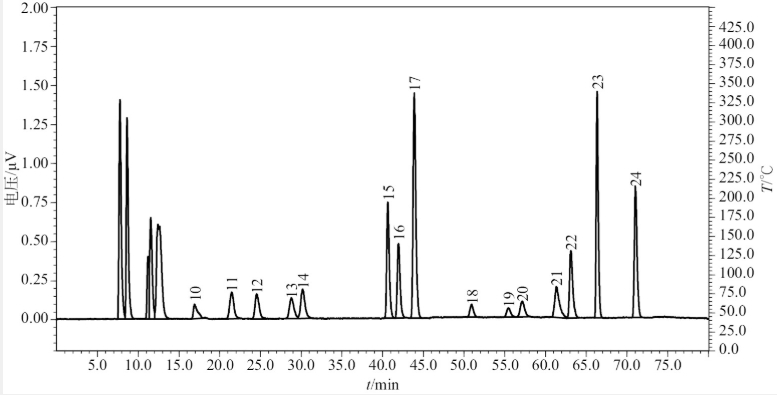
Fig 1.1 HPLC-ELSD chromatogram of Mixed Standard Solution A
10-Cys-Cys; 11-Pro;12-2-Gamma-Aminobutyric Acid;13-Lys;14 His;15-Val; 16-DL-3,4-DOPA;17-Arg; 18Met;19-Tyr;20-lle;21-Leu;22-Nle;23-Phe;24-Trp
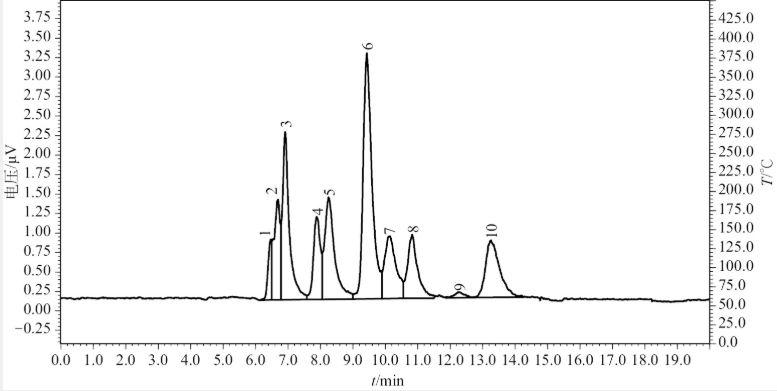
Fig 1.2 HPLC-ELSD chromatogram of Mixed Standard Solution B
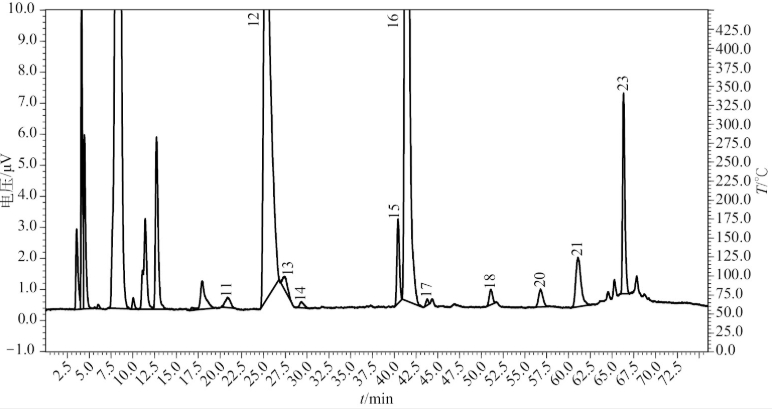
Fig 1.3 HPLC-ELSD chromatogram of amino acids from Wangqing sexennial Panax ginseng
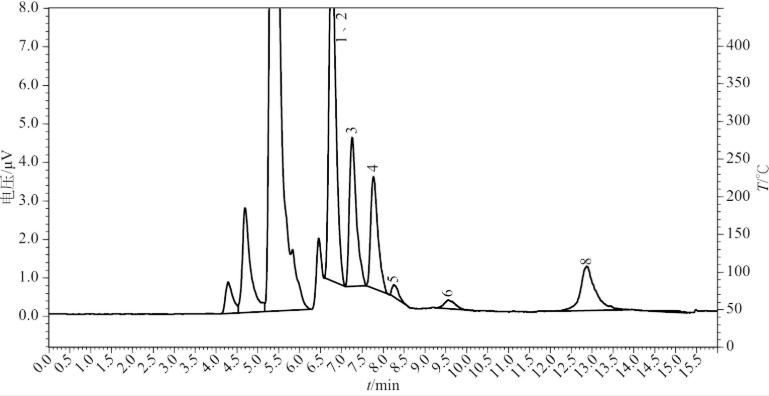
Fig 1.4 HPLC-ELSD chromatogram of amino acids from Kuandian quinguennium Panax ginseng


